|
AUTOZINE TECHNICAL SCHOOL
Emission Control Pollutants and Emission Standards
Ideally, an engine combusts fossil fuel and air into CO2 and H2O. In reality, no matter how clean an engine combusts, it still produces some by-products, i.e. pollutants. The key pollutants are:
To deal with the pollution problems, US mandated the first nation-wide emission standard in 1975. Europe and Japan passed similar legislations in the early 1990s. Over the years, emission standards have been tightening step by step. For example: EU standards for gasoline passenger cars:
EU standards for diesel passenger cars:
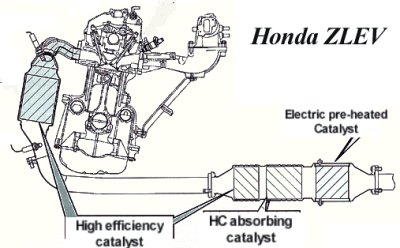
(Unit: mg/km) It is increasingly likely that gasoline and diesel emission standards will be unified into a common standard in the future. Catalytic Converter The Federal emission standard US enforced in 1975 was so stringent that car makers had to install catalytic converter to every car sold there (remark: the only exception was Honda Civic, whose CVCC technology managed to comply without using catalytic converter). A catalytic converter is installed at the downstream of the exhaust system. It uses catalysts such as platinum, rhodium and palladium to convert NOx into nitrogen and oxygen. The oxygen then reacts with CO to become CO2, or reacts with HC to become CO2 and H2O. These chemical reactions may happen in natural environment, but the catalyst accelerates the process massively thus is capable to treat most of the pollutants emitted. On the downside, the presence of catalytic converter slows down the exhaust flow and creates back pressure, reducing engine output. That is why when Euro 1 standard was enforced in 1993, we saw most European cars lost at least several horsepower. Catalytic converters have their limitations. One of which is sulphur pollution. Sulphur inherently existed in fuel could pollute some precious metal catalyst and render the latter useless. This problem was finally solved when governments mandated oil companies to supply low-sulphur fuel. Another problem is warm up time. Catalyst needs at least 400°C to function effectively. When the car starts from cold, it takes time for the exhaust gas to heat up the exhaust system and bring up the catalyst to this temperature. Durng this period, the car emits high level of pollutants. As newer emission standards measure also cold-start emission, car manufacturers have to search for solutions. Popular solutions include close-coupled catalyst, HC traps and electrically heated catalyst.
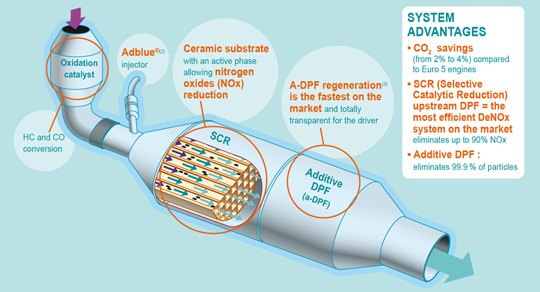
Diesel Particulate Filter (DPF) Particulate emission is always the biggest headache of diesel engines. These particulates are composed of mainly carbon and hydrocarbons. They lead to black smoke and smog which is critical to the air quality of urban areas. Small particulate matters (PM2.5 or below) may penetrate into lungs and cause serious health problems. Therefore, lower and lower PM limits were imposed in the past 2 decades for diesel car emission. To deal with tighter requirements, PSA group developed the first mass production diesel particulate filter in 2000. It was first used on the company's 2.2 HDi engine on Peugeot 607. By 2009, all its diesel cars were already equipped with DPF. Basically, PSA’s DPF is a porous silicon carbide unit, comprising of passageways which have a property easily trap and retain particulates from the exhaust gas flow. Before the filter surface is fully occupied, these particulates should be burnt up, becoming CO2 and water and leaving the filter accompanied with exhaust gas flow. We call this process as regeneration. Normally, regeneration takes place at a temperature around 550°C. Problem is, such a high temperature is not obtainable in reality. The exhaust gas of diesel engines is always cooler than gasoline engines. When the car is stuck in traffic or driving slowly in town, its exhaust gas temperature may drop to between 150 and 200°C. 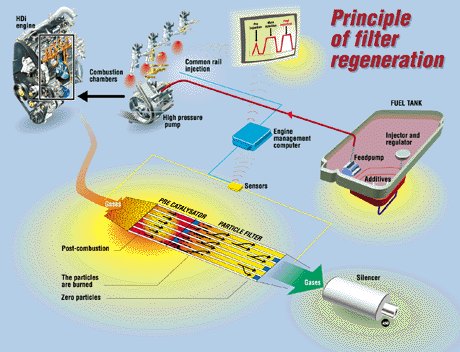 Fortunately, the new common-rail injection technology employed by PSA's diesel engines helped. Gifted by its high-pressure, precise injection during a very short period, the common-rail system can introduce a "post-combustion" by injecting a small amount of fuel during expansion phase. This increases the exhaust flow temperature to around 350°C. Then, a specially designed oxidizing catalyst located near the entrance of the particulate filter unit combusts the remaining unburnt fuel coming from the post-combustion. This raises the temperature further to 450°C. The last 100°C required is fulfilled by adding an additive called Eolys to the fuel. Eolys lowers the operating temperature of particulate burning to 450°C, now regeneration occurs. The liquid-state additive is store in a small tank and added to the fuel by pump. The DPF unit needs to be cleaned at dealerships every 80,000 km by high-pressure water in order to get rid of the deposits resulting from the additive. One more thing to be solved is the influence of "post-combustion". It increases engine torque when the driver doesn’t expect. Therefore the engine management system has to regulate the torque by adjusting the amount of normal fuel injection, pre-injection etc. and turbocharger’s boost pressure to compensate. Selective Catalytic Reduction (SCR) Apart from particulate matter, diesel engines also emit higher levels of NOx than gasoline counterparts, since NOx is more likely to be formed under conditions of lean mixture and high compression. As Euro 6 and US Tier 2 emission standards have NOx limits tightened significantly, most diesel engines have to seek help from Selective Catalytic Reduction, which is capable to reduce NOx by 90 percent.  SCR converter injects a urea solution,
commercially known as AdBlue, to the exhaust stream. It reacts with NOx
with the aid of catalyst (coated on substrate), resulting in nitrogen
and water vapour. The AdBlue is stored in a dedicated tank and can be
refilled, normally every several thousand miles.
 Exhaust Gas Recirculation (EGR) Exhaust Gas Recirculation is another technology to reduce NOx emission. In fact, it had been widely used in diesel engines well before the popularity of catalytic converters. 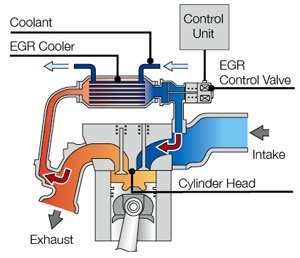 A typical EGR system adds a recirculation
pipe to bridge between intake and exhaust manifolds, drawing some
exhaust gas back into the combustion chamber. A valve in the pipe
controls the amount of EGR. The pipe may be added with a liquid cooler
to cool the exhaust gas, resulting in higher efficiency. A typical EGR system adds a recirculation
pipe to bridge between intake and exhaust manifolds, drawing some
exhaust gas back into the combustion chamber. A valve in the pipe
controls the amount of EGR. The pipe may be added with a liquid cooler
to cool the exhaust gas, resulting in higher efficiency. Another possible mechanism is simpler. It uses variable valve timing to delay the closure of exhaust valves, so that some exhaust gas is drawn back from the exhaust manifold during the intake stroke. This is called "internal EGR". How can EGR reduce NOx emission? As the combustion chamber is now filled with less fresh air and some exhaust gas, which consists of mostly nitrogen, CO2 but little oxygen, the total oxygen content is reduced. This means less fuel injection is required. With less fuel and oxygen, the combustion results in less power and heat. The corresponding lower temperature leds to less NOx generated. Another reason for the lower combustion temperature is that the mixture of more nitrogen and less oxygen results in a higher specific heat capacity (energy needed to heat up the material by 1K), which means the mixture can absorb more energy. However, it must be noted that some modern gasoline engines also use EGR, not to cut NOx emission (which is inherently lower than diesel engines), but to improve fuel efficiency. One example is Toyota 1NR-FKE 1.3-liter engine. Running EGR can save fuel because:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Copyright© 1997-2016 by Mark Wan @ AutoZine |